Why Does Temp Increase With Pressure . The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This is a temperature scale where temperature and pressure are. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \).
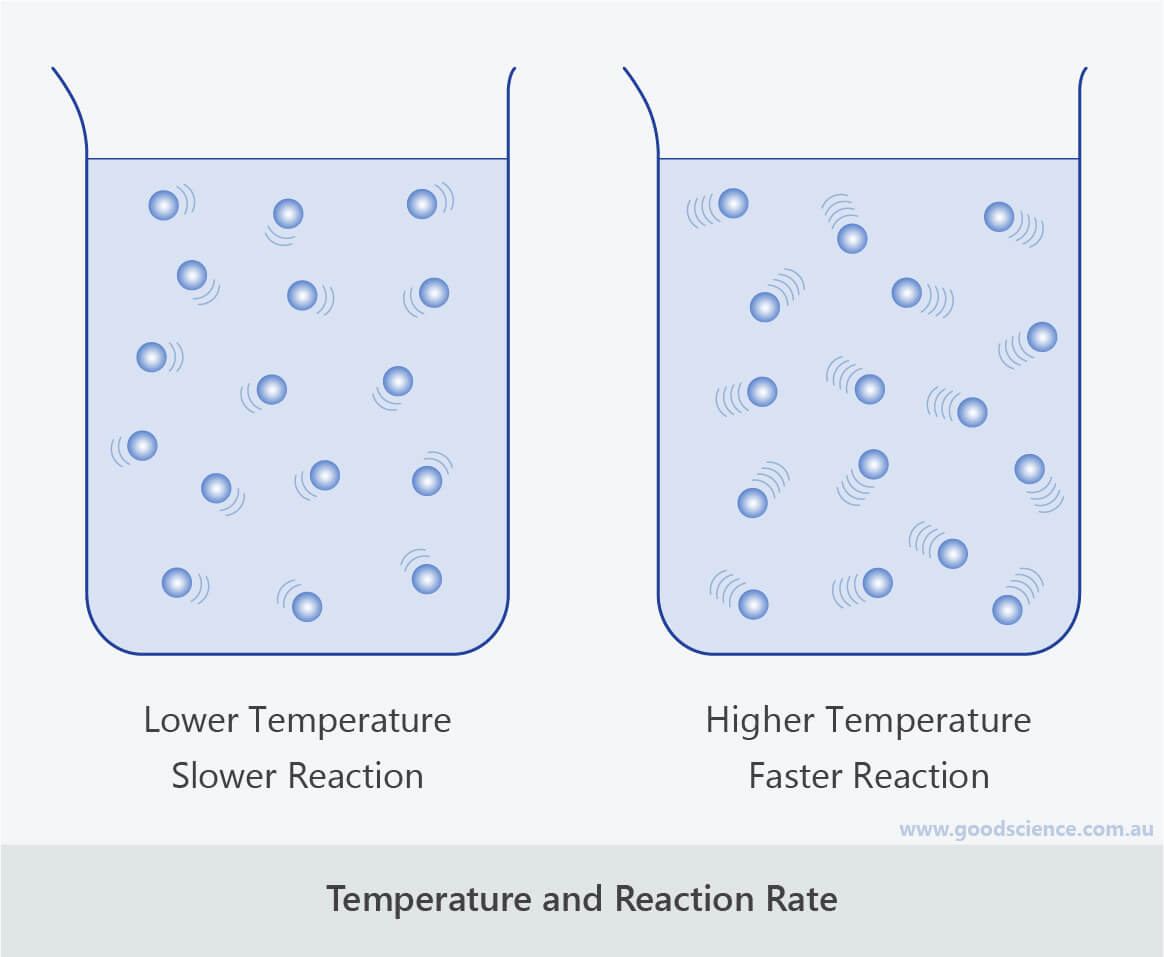
from www.goodscience.com.au
The energy added as work during the compression of a gas leads to an increase in pressure and temperature. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. This is a temperature scale where temperature and pressure are. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas.
Factors that Affect Rate of Reaction Good Science
Why Does Temp Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure are.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID1757364 Why Does Temp Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. The energy added as work during the compression of a gas leads to an increase in. Why Does Temp Increase With Pressure.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Why Does Temp Increase With Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The energy added as work during the compression of a gas leads to an increase in pressure. Why Does Temp Increase With Pressure.
From pressbooks.bccampus.ca
LABORATORY 2 HEAT AND TEMPERATURE IN THE ATMOSPHERE Physical Why Does Temp Increase With Pressure This is a temperature scale where temperature and pressure are. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The relationships among the volume of a gas and its. Why Does Temp Increase With Pressure.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Why Does Temp Increase With Pressure We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. If you had a way to increase pressure with. Why Does Temp Increase With Pressure.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Why Does Temp Increase With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. This is a. Why Does Temp Increase With Pressure.
From socratic.org
A given mass of oxygen at room temperature occupies a volume of 500.0 Why Does Temp Increase With Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure are. The energy added as work during the compression of a gas leads to. Why Does Temp Increase With Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Why Does Temp Increase With Pressure To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). This is a temperature scale where temperature and pressure are. We find that temperature and. Why Does Temp Increase With Pressure.
From www.tec-science.com
Why do pressure and temperature increase during the compression of a Why Does Temp Increase With Pressure This is a temperature scale where temperature and pressure are. If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. The energy added as work during the compression of a gas leads to an increase in pressure and temperature. A temperature increase of \(1k\) = a temperature increase of. Why Does Temp Increase With Pressure.
From www.slideserve.com
PPT Unit 4 Phases of Matter (Chapters 1314) PowerPoint Presentation Why Does Temp Increase With Pressure We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. This is a temperature scale where temperature and pressure. Why Does Temp Increase With Pressure.
From www.youtube.com
Partial Pressure Change with Temperature (Interactive) YouTube Why Does Temp Increase With Pressure This is a temperature scale where temperature and pressure are. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. We find that temperature and pressure are linearly related, and. Why Does Temp Increase With Pressure.
From www.researchgate.net
Variations of the pressure increase, temperature rise, and film Why Does Temp Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea. Why Does Temp Increase With Pressure.
From chem.libretexts.org
13.4 Effects of Temperature and Pressure on Solubility Chemistry Why Does Temp Increase With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. This is a temperature scale where temperature and pressure are. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). To get some idea of how pressure, temperature, and volume of a. Why Does Temp Increase With Pressure.
From gcsephysicsninja.com
11. Heating gas at a constant pressure Why Does Temp Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea of how pressure, temperature, and volume of a gas are related to. Why Does Temp Increase With Pressure.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Why Does Temp Increase With Pressure A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). This is a temperature scale where temperature and pressure are. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then. Why Does Temp Increase With Pressure.
From www.nagwa.com
Question Video Determining How a Change in Temperature Affects the Why Does Temp Increase With Pressure The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). If you had a way to increase pressure with no volume change, then yes, temperature would increase by the ideal gas. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p. Why Does Temp Increase With Pressure.
From garciaspeausell.blogspot.com
Why Did the Temperature Rise to a Constant Temperature Instead of Why Does Temp Increase With Pressure We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. The relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). A temperature increase of \(1k\) = a temperature increase of \(1^{\circ}c \). To get some idea. Why Does Temp Increase With Pressure.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Does Temp Increase With Pressure The energy added as work during the compression of a gas leads to an increase in pressure and temperature. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. To get some idea of how pressure, temperature, and volume of a gas are related to. Why Does Temp Increase With Pressure.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Why Does Temp Increase With Pressure To get some idea of how pressure, temperature, and volume of a gas are related to one another, consider what happens when you pump air into. We find that temperature and pressure are linearly related, and if the temperature is on the kelvin scale, then p and t are directly proportional. This is a temperature scale where temperature and pressure. Why Does Temp Increase With Pressure.